Enhancing Reproductive Performance in Small Ruminants: Part V. Nutrition and Health
ID
APSC-164P (APSC-236P)
EXPERT REVIEWED
This series of fact sheets has been designed to assist producers in enhancing reproductive performance in their herd so that overall production can be optimized to promote profitability. Fact sheet topics included in the Enhancing Reproductive Performance in Small Ruminants series include:
Part I. Biology of Reproduction Part II. Puberty and Estrous Cycles
Part III. Breeding and Management Systems Part IV. Breed/Selection
Part V. Nutrition and Health
Part VI. Reproductive Management Techniques
Nutrition
Herd nutrition has a direct influence on reproductive performance, and of all the issues discussed, this is the one factor that the producer has the most control over. Strategic use of nutritional supplements is known to improve a number of reproductive traits, including enhancing the breeding condition of males, maximizing ovulation rate to increase litter size, reducing early embryo loss, and maximizing postnatal survival and development. A good plane of nutrition can also decrease the length of the post-partum interval (the time between kidding and re-breeding).
A relatively easy method of determining if sufficient nutrition is being provided is to conduct body condition scoring (BSC) (figure 1). Every producer should be familiar with how to assess BCS. Langston University has a great reference on how to correctly do BCS in goats available online at https://goats.extension.org/goat-body-condition-score/.
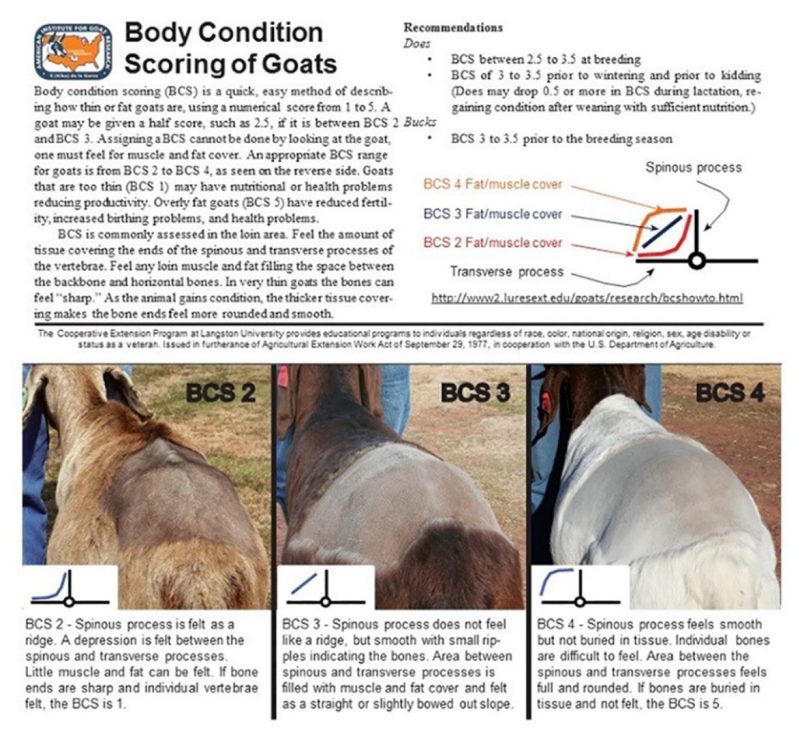
All animals, including males, should be evaluated for adequate BCS two months before the breeding season. An optimal BCS of 3 is desired, and if most animals are below this, feed needs to be adjusted for animals to meet the required BCS before breeding.
Improving nutrition before breeding to boost body condition can also increase the number of ovulations per female. This is referred to as flushing (figure 2). Adjusting nutrition should begin three to four weeks before breeding and continued through the first three weeks of breeding. Generally, results from flushing vary. It is most beneficial if used early in the breeding season and when there are a significant number of thin females (those with a BCS less than 2.5).

Adequate nutrition is important during the first 90 days of pregnancy, especially for placental development (figure 3). However, only a small increase relative to what is required for maintenance is needed. Inadequate feeding can result in reduced survival rates at birth. All pregnant females should maintain a BCS of 3 during early gestation and onwards. If this cannot be maintained on pasture or with hay, supplemental feed might be required.

Excess losses in body weight during pregnancy can place females at risk for developing pregnancy toxemia. Pregnancy toxemia is a metabolic disease resulting from the production of ketones in the body due to inadequate intake of carbohydrates, leading to the breakdown of fat. It is generally seen in females that are obese, have multiple fetuses, or are underfed. Nutrition during the last four to six weeks is very crucial, as approximately two- thirds of fetal growth happens at this stage. Most of the female’s mammary (udder) development is occurring as well, and under-feeding can affect subsequent colostrum and milk production. Inadequate nutrition can also lead to abortions and lower-than-normal birth weights in kids and lambs. On the other hand, overfeeding results in obesity, contributing to dystocia, and it puts females at an increased risk for developing pregnancy toxemia. Therefore, it is very important that feeding regimes minimize the energy being supplied by body fat reserves and strive to maintain the flock at an optimum BCS. Grain supplementation is usually necessary to meet increased energy demands, especially when forage quality is low and when females are high-producing. The amount of grain supplementation needed will depend on the quantity and quality of available forage, breed, the number of fetuses, doe size, and age.
Because adequate nutrition is critical, it is important to ensure sufficient feed bunk space for pregnant and lactating does and ewes. Some females, especially small or young ones, might not get enough to eat, and it might be necessary to feed them separately. Feeding on the ground should always be avoided due to potential spread of infectious abortive diseases. Higher-producing females should be fed higher-quality feed, especially those nursing twins and triplets. During pregnancy and lactation, free choice minerals might not ensure adequate intake, especially of calcium. Calcium requirements generally increase during early and late gestation and peak during lactation. Additionally, calcium requirements are highest for females carrying multiple fetuses and those producing more milk. For instance, a 125-pound ewe requires approximately 0.005 pounds of calcium per day for maintenance. For a similar-sized female carrying twins, the daily calcium requirement increases to 0.01 and 0.02 pounds of calcium per day during late gestation and lactation, respectively. Feed rations should be evaluated, and any adjustments should be made to ensure that sufficient calcium is provided at these times to avoid an outbreak of milk fever.
Health
The overall objective of a good health management plan that enhances reproductive efficiency is the successful completion of pregnancy; birth of healthy, strong offspring; optimal birth and weaning weights; and optimum milk production. A good health management plan for ewes and does should include adequate nutrition (described above), udder health, internal parasite management, vaccinations, and the prevention of abortive and metabolic disorders (pregnancy toxemia and hypocalcemia) (figure 4).
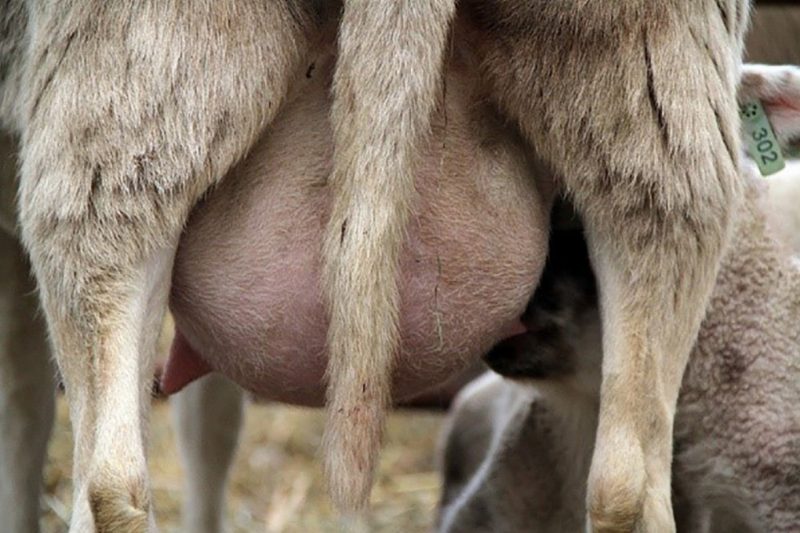
Effective udder health management includes careful examination by palpation for any hardness, abscesses, or nodules. It has been documented that mammary infections increase during the weeks following the cessation of lactation. Evaluating the mammary glands at this time will help to identify any females with abnormalities that should be culled, especially since kids and lambs from dams with mammary gland abnormalities tend not to thrive as well as those from dams with healthy udders.
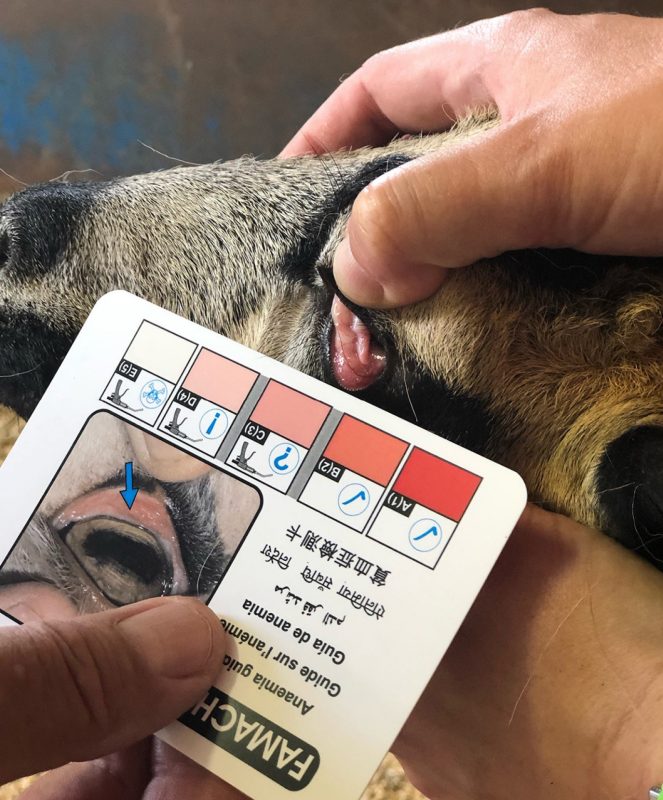
Heavy infections with internal parasites can reduce the BCS of breeding females and may reduce reproductive performance in the herd. To minimize any negative effects, a regular parasite control program must be implemented. Goat and sheep producers should be using tools such as the FAMACHA© system (figure 5) and Five Point Check© in a targeted selective treatment approach. This is especially important around the time of kidding and lambing when females experience a decline in their normal immunity to internal parasites and can experience a periparturient rise in fecal egg counts (an increase in the number of parasite eggs around the time of delivery).
These parasite eggs then become the primary source of parasite infection for new offspring, who are highly susceptible to infections due to their naïve immune systems. As expected, kidding/lambing on pasture (winter and spring) and having younger females in the flock increases this risk. Selective deworming using FAMACHA© and the Five Point Check© two to four weeks prior to kidding/lambing helps to kill parasites and reduce pasture infestation. The suppression of the periparturient rise in fecal egg counts depends on the effectiveness of the dewormer used. The American Consortium for Small Ruminant Parasite Control (ACSRPC) currently recommends that animals showing clinical signs of parasitism be dewormed with a combination treatment. That is, the animal should be treated with the most potent drug from each class (albendazole, moxidectin and levamisol) sequentially to increase efficacy and reduce the development of drug resistance. For more information on small ruminant parasites, prevention and treatment, visit www.wormx.info. Finally, the impact of good nutrition, especially protein and trace minerals needed to support a strong immune response, should never be underestimated in an effective parasite control program. Research has shown that ewes receiving higher levels of protein (greater than 14%) for six weeks prior to lambing have significantly lower fecal egg counts.
A good health management plan aims to ensure health of pregnant females as well as their offspring. Vaccination against diseases caused by Clostridium perfringens, including enterotoxemia (known as overeating disease or pulpy kidney disease; caused by C. perfringens Type D), bloody scours (Type C), and tetanus (lockjaw; Type D), are generally effective, and all females should be vaccinated two to four weeks prior to giving birth.
This allows dams to provide passive immunity to their offspring through colostrum. The antibodies from colostrum can only be absorbed within the first 24 hours after birth, and it is critical that kids and lambs nurse soon after being born so that they are protected against these diseases. This passive immunity will last for approximately six weeks in kids and lambs. At around this time, they should be given the first injection of the vaccine, followed by a booster two to four weeks later.
Small ruminants are also susceptible to infectious abortive diseases such as chlamydia, toxoplasmosis, brucellosis, listeriosis, Q fever, and a host of others (figure 6). A few of these have vaccinations available, and if there is a history of abortions or weak, small kids/ lambs born in a herd, the cause should be diagnosed.
Detailed history, blood tests and/or isolation of bacteria from placenta or fetal tissue can be used to accurately diagnose an infectious abortive disease in a herd. If vaccination was not carried out or is not available, feeding chlortetracycline (Aureomycin at a rate of 80 milligrams/head/day during last six weeks of gestation), or administering injections of antibiotics (LA-200; oxytetracycline) at two-week intervals during last six weeks of gestation have been shown to be effective in preventing abortions. Be advised that the Veterinary Feed Directive was modified to regulate how antibiotics that are important for treating humans are used in water or feed as a treatment for animals. Therefore, it is now required that a veterinarian supervise the administration of these antibiotics in feed or water to food animal species.

Abortions due to toxoplasmosis can be prevented by feeding a coccidiostat in the feed six weeks before lambing/kidding. Coccidiostats are not affected by the new feed directive regulations, as they are not considered medically important to humans. It is important to note also that pregnant women should never handle aborted material due to the risks to their own pregnancy. For instance, Chlamydia abortus, the agent responsible for causing chlamydia, is zoonotic (spread from animals to humans) and can cause serious health problems in pregnant women.
Acknowledgements
The authors would like to acknowledge Kwame Matthews (Delaware State University), Gabriel Pent (Virginia Tech), James Hilleary (Virginia Cooperative Extension) and Cynthia Martel (Virginia Cooperative Extension) for their time in reviewing all fact sheets in this series.
Additional Resources
Barkema, H. W., Y. H. Schukken, T. J. G. M. Lam, M. L. Beiboer, H. Wilmink, G. Benedictus, and A. Brand. 1998. “Incidence of Clinical Mastitis in Dairy Herds Grouped in Three Categories by Bulk Milk Somatic Cell Counts.” Journal of Dairy Science 81:411–419. https://doi.org/10.3168/jds.S0022-0302(98)75591-2.
Donaldson, J., M. F. J. van Houtert, and A. R. Sykes. 1997. “The Effect of Protein Supply on the Periparturient Parasite Status of the Mature Ewe.” Proceedings of the New Zealand Society of Animal Production 57: 186-189. http://www.nzsap.org/system/files/proceedings/1997/ab97052.pdf.
Fthenakis, G. C., G. Arsenos, C. Brozos, I. A. Fragkou, N. D. Giadinis, I. Giannenas, V. S. Mavrogianni, E. Papadopoulos, and I. Valasi. 2012. “Health Management of Ewes During Pregnancy.” Special Issue: Reproductive Health Management of Sheep and Goats. Animal Reproduction Science 130 (3-4): 198-212.
Martin, G. B., J. T. B. Milton, R. H. Davidson, G. E. Banchero Hunzicker, D. R. Lindsay, and D. Blache. 2004. Natural Methods for Increasing Reproductive Efficiency in Small Ruminants. Animal Reproduction Science 82–83 (July 2004): 231–245.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
October 23, 2025



